![]() +44 207 488 9947
+44 207 488 9947












Antimicrobial Resistance (AMR) in Human Medicine
Elite antimicrobial 
resistance lawyers
advising on the legal
and regulatory aspects
of R&D to the supply
of novel antimicrobial
medicines and vaccines
The leading antimicrobial resistance lawyers will advise and assist you with your initiatives to develop, manufacture and supply antimicrobial medicines. The antimicrobial resistance multidisciplinary team at our law firm comprises scientists with first-hand experience of conducting research as well as working in the pharmaceutical and biopharmaceutical industry sector. Our antimicrobial resistance team is well placed to guide you on legal and regulatory issues that may arise during the development of novel antimicrobial medicines and vaccines aimed at limiting resistance or reducing the emergence and spread of antimicrobial resistance.
Antimicrobial resistance occurs when microbes evolve to become more or fully resistant to antimicrobials which previously could treat them such as antibiotics. As pathogens become resistant to antimicrobials the chances of treating infections are substantially reduced. Our AMR team is cognisant of the urgent threat of antimicrobial resistance, posed by the emerging and steady increase of microbes that are resistant to antimicrobial treatments including the threat to the effective treatment of infectious diseases. Accordingly, our antimicrobial resistance lawyers have the knowledge and expertise to advise and assist you and to provide the highest quality legal and regulatory advice whether you are a research-based pharmaceutical company or a biotechnology company; once you are involved in the development of innovative new medicines, new diagnostic tests and/or utilising antibacterial clinical development.
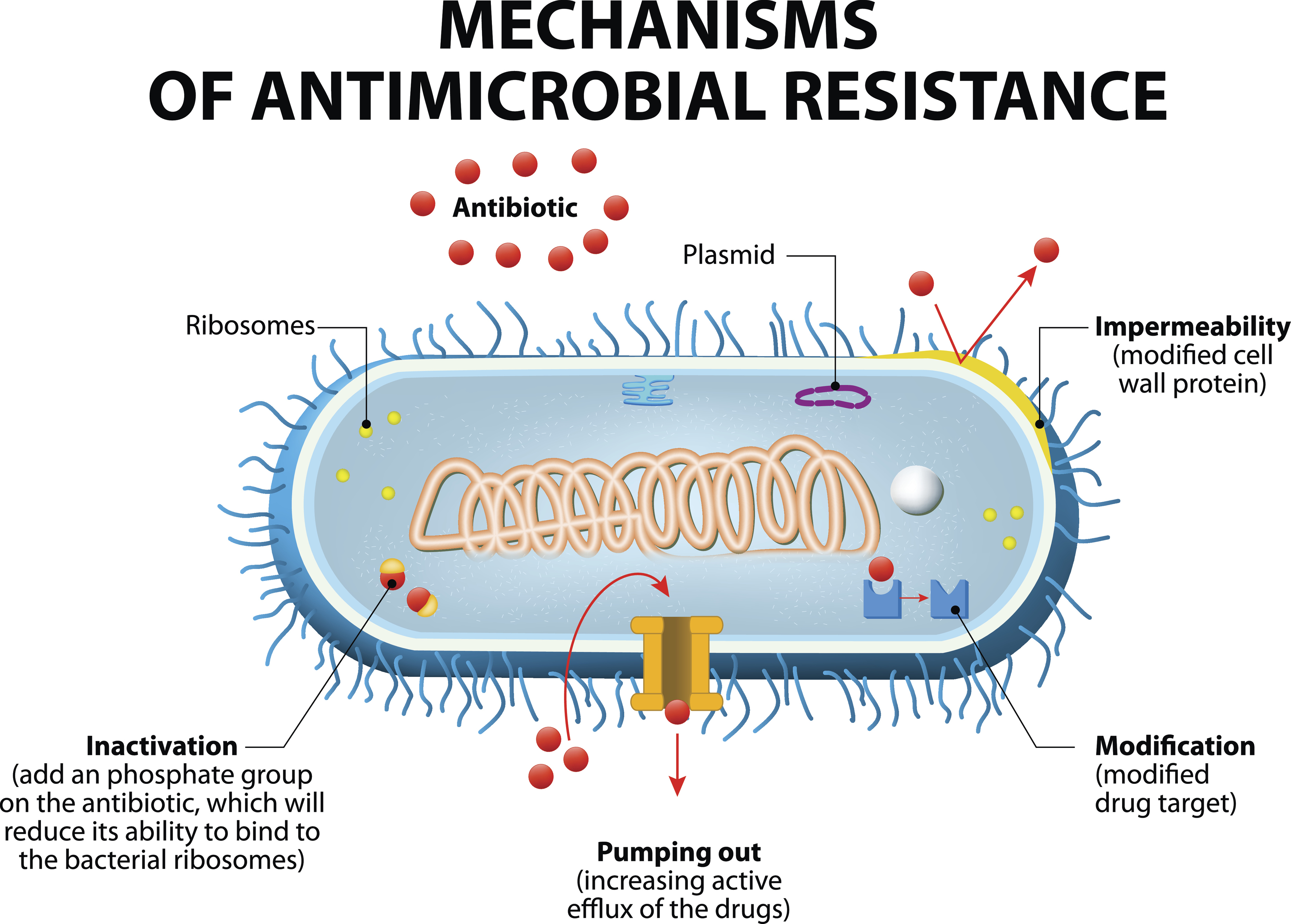
The antimicrobial resistance team comprises lawyers and scientists with doctorates and post doctorates and as such appreciate that as existing antimicrobial medicines become obsolete, due to resistance, you continue to research and develop novel antimicrobial medicines and vaccines.
Innovation, Research & Development, Development of Treatments and Therapies
- Antibacterial Clinical Development
- Preclinical and Clinical Data
- Antimicrobial Medicines
- Bacterial and Fungal Infections
- Antimicrobial R&D
- Development of New Medicines and Treatment
- Drug development for Novel Antibacterial Agents
- Non-Antibiotics and Antibiotics
- Anti-Virulence Agents
- Biological Agents
- Combination Therapies
- Healthcare Delivery Systems
- Innovation in the Development of New Antibiotics
- New Diagnostic Tests
- Monoclonal Antibodies and Vaccines on Antimicrobial Resistance
- Development of Mathematical Models
- Vaccines.
Our Services
Our legal and regulatory services in connection with antimicrobial resistance include:
| Artificial Intelligence |
Advise and Assist with:
|
| Clinical Trials |
|
|
Commercial Contracts, Licensing, Joint Ventures, Mergers and Acquisitions |
|
| Dossier |
|
|
|
| Manufacturing |
|
| Regulatory Compliance: Marketing, Selling, Offer for Sale or Supplying Products |
|
| Testing |
|
Related Areas
- Agrochemicals
- CBD: Cannabidiol Products
- Clinical Trials
- Commercial Contracts
- Corporate Finance
- Diseases
- Herbal Medicines
- Household & Consumer Product Regulatory
- Innovative Medicine
- Intellectual Property
- Life Sciences
- Medical Devices
- Pharmaceuticals
- Plant Protection Products
- Vaccines
- Virtual R&D
Testimonials
 Ice Healthcare Ltd
Ice Healthcare Ltd
“RT Coopers Solicitors operate in the real world and deliver what they say they’ll deliver. A highly responsive, straight talking, client focused team who will pull out all the stops to deliver on-time results of the highest standard. Definitely a team you want along side you when the going gets tough. I think that says it all…...” Janet Dean, Operations Director
For more Testimonials on life sciences and biotechnology, pharmaceuticals and antimicrobial resistance.
![]()
- GPhC Extension to Registration
24-08-2025 - Testimonial: Appeal to GPhC
17-01-2025 - Practice Area: Social Media Law
14-01-2025 - Practice Area: AI & Data Science
15-12-2024 - Case Law: Churchill Case
30-11-2024 - Testimonial : Academic Misconduct
23-11-2024 - Testimonial - MPharm Student
22-09-2024 - MODERNA V. PFIZER/BIONTECH
28-08-2024 - Patents - Artificial Intelligence
05-07-2024 - Case Study : OSCE Retake
26-06-2024 - IBM vs LZLABS report by D Corbin
20-05-2024 - Case Study : Biomedical Science
14-03-2024 - Case study : MSc Nursing Student
22-02-2024 - Case Study : Accused of Plagiarism
09-11-2023 - Testimonial : MBBS Resit Failure
05-11-2023
![]()
![]()
![]()
![]()
![]()


